Online first
Bieżący numer
O czasopiśmie
Archiwum
Polityka etyki publikacyjnej
System antyplagiatowy
Instrukcje dla Autorów
Instrukcje dla Recenzentów
Rada Redakcyjna
Bazy indeksacyjne
Komitet Redakcyjny
Recenzenci
2024
2023
2022
2021
2020
2019
2018
Kontakt
Klauzula przetwarzania danych osobowych (RODO)
PRACA POGLĄDOWA
Wpływ zanieczyszczeń powietrza i metali ciężkich na ryzyko wystąpienia choroby trzewnej (celiakii) w populacji pediatrycznej
1
Student’s Research Group, Medical University, Lublin, Poland
Autor do korespondencji
Kacper Bartosik
Studenckie Koło Naukowe Uniwersytetu Medycznego w Lublinie, Uniwersytet Medyczny w Lublinie, al. Racławickie, 20-059, Lublin, Polska
Studenckie Koło Naukowe Uniwersytetu Medycznego w Lublinie, Uniwersytet Medyczny w Lublinie, al. Racławickie, 20-059, Lublin, Polska
Med Srod. 2025;28(3):108-114
SŁOWA KLUCZOWE
odpowiedź immunologicznametale ciężkiezanieczyszczeniapowietrzachoroba trzewnaczynniki środowiskowedzieci
DZIEDZINY
STRESZCZENIE
Wprowadzenie i cel:
Celiakia to przewlekła choroba autoimmunologiczna jelita cienkiego, rozwijająca się u osób z predyspozycją genetyczną (HLA-DQ2/DQ8) po ekspozycji na gluten. Mimo że czynniki genetyczne odgrywają kluczową rolę w jej patogenezie, obserwowany w ostatnich dekadach wzrost liczby zachorowań sugeruje wpływ także czynników środowiskowych. Celem pracy było przeanalizowanie najnowszych danych dotyczących wpływu zanieczyszczeń powietrza oraz ekspozycji na metale ciężkie na ryzyko rozwoju i przebieg choroby trzewnej u dzieci i młodzieży.
Opis stanu wiedzy:
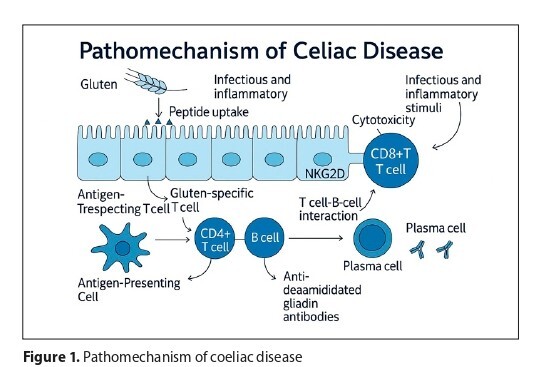
Coraz więcej badań wskazuje, że metale ciężkie mogą odgrywać rolę w powstaniu i nasileniu celiakii. Nanocząstki srebra, tlenku tytanu i złota zaburzają mikrobiom jelitowy, uszkadzają barierę śluzówkową i aktywują układ odpornościowy, zwiększając poziom cytokin prozapalnych (m.in. IL-15, IFNγ, IL-8). U chorych obserwuje się nadmiar bakterii z rodzaju Bacteroides i Firmicutes, co może być skutkiem ekspozycji na metale. Dodatkowo związki te upośledzają integralność połączeń między komórkami nabłonka i zaburzają autofagię w enterocytach, sprzyjając destrukcji jelita cienkiego. Osoby na diecie bezglutenowej są też bardziej narażone na akumulację metali (np. z ryżu), co może nasilać uszkodzenia. Zanieczyszczenia powietrza, takie jak NO₂, pyły PM i ozon, także uszkadzają barierę jelitową, wpływają na odporność i zwiększają ryzyko celiakii. Wśród dzieci choroba trzewna częściej diagnozowana jest u tych, które mieszkają w rejonach o większym zanieczyszczeniu.
Podsumowanie:
Metale ciężkie i zanieczyszczenia powietrza mogą wpływać na mikrośrodowisko jelitowe, barierę nabłonkową i odpowiedź immunologiczną, stanowiąc potencjalne czynniki ryzyka rozwoju celiakii. Ich rola w patogenezie tej choroby wymaga dalszych, wieloośrodkowych badań.
Celiakia to przewlekła choroba autoimmunologiczna jelita cienkiego, rozwijająca się u osób z predyspozycją genetyczną (HLA-DQ2/DQ8) po ekspozycji na gluten. Mimo że czynniki genetyczne odgrywają kluczową rolę w jej patogenezie, obserwowany w ostatnich dekadach wzrost liczby zachorowań sugeruje wpływ także czynników środowiskowych. Celem pracy było przeanalizowanie najnowszych danych dotyczących wpływu zanieczyszczeń powietrza oraz ekspozycji na metale ciężkie na ryzyko rozwoju i przebieg choroby trzewnej u dzieci i młodzieży.
Opis stanu wiedzy:
Coraz więcej badań wskazuje, że metale ciężkie mogą odgrywać rolę w powstaniu i nasileniu celiakii. Nanocząstki srebra, tlenku tytanu i złota zaburzają mikrobiom jelitowy, uszkadzają barierę śluzówkową i aktywują układ odpornościowy, zwiększając poziom cytokin prozapalnych (m.in. IL-15, IFNγ, IL-8). U chorych obserwuje się nadmiar bakterii z rodzaju Bacteroides i Firmicutes, co może być skutkiem ekspozycji na metale. Dodatkowo związki te upośledzają integralność połączeń między komórkami nabłonka i zaburzają autofagię w enterocytach, sprzyjając destrukcji jelita cienkiego. Osoby na diecie bezglutenowej są też bardziej narażone na akumulację metali (np. z ryżu), co może nasilać uszkodzenia. Zanieczyszczenia powietrza, takie jak NO₂, pyły PM i ozon, także uszkadzają barierę jelitową, wpływają na odporność i zwiększają ryzyko celiakii. Wśród dzieci choroba trzewna częściej diagnozowana jest u tych, które mieszkają w rejonach o większym zanieczyszczeniu.
Podsumowanie:
Metale ciężkie i zanieczyszczenia powietrza mogą wpływać na mikrośrodowisko jelitowe, barierę nabłonkową i odpowiedź immunologiczną, stanowiąc potencjalne czynniki ryzyka rozwoju celiakii. Ich rola w patogenezie tej choroby wymaga dalszych, wieloośrodkowych badań.
Introduction and objective:
Coeliac disease is a chronic autoimmune disorder of the small intestine that develops in genetically predisposed individuals (HLA-DQ2/DQ8) following gluten exposure. Although genetic factors play a pivotal role in its pathogenesis, the rising incidence observed over recent decades suggests that environmental factors also contribute. The aim of the review is to analyze recent data regarding the influence of air pollution and heavy metal exposure on the risk and course of coeliac disease in children and adolescents.
Brief description of the state of knowledge:
An increasing number of studies indicate that heavy metals and air pollutants may contribute to the development and progression of coeliac disease. Nanoparticles of silver, titanium dioxide, and gold disrupt the gut microbiota, damage the intestinal barrier, and activate the immune system, elevating pro-inflammatory cytokines such as IL-15, IFN-γ, and IL-8. Exposure to heavy metals is associated with over-representation of Bacteroides and Firmicutes, impaired tight junctions, and disrupted autophagy in enterocytes, all promoting intestinal injury. Individuals on gluten-free diets, especially those rich in rice-based products, may accumulate more heavy metals, exacerbating mucosal damage. Likewise, pollutants like nitrogen dioxide, particulate matter, and ozone weaken gut integrity, alter immune responses, and are linked to increased coeliac disease prevalence, particularly in children in polluted areas.
Summary:
Heavy metals and air pollution may alter the intestinal microenvironment, epithelial barrier function, and immune response, representing potential risk factors for the development of coeliac disease. Their role in the disease’s pathogenesis warrants further multicentre, multidisciplinary research.
Coeliac disease is a chronic autoimmune disorder of the small intestine that develops in genetically predisposed individuals (HLA-DQ2/DQ8) following gluten exposure. Although genetic factors play a pivotal role in its pathogenesis, the rising incidence observed over recent decades suggests that environmental factors also contribute. The aim of the review is to analyze recent data regarding the influence of air pollution and heavy metal exposure on the risk and course of coeliac disease in children and adolescents.
Brief description of the state of knowledge:
An increasing number of studies indicate that heavy metals and air pollutants may contribute to the development and progression of coeliac disease. Nanoparticles of silver, titanium dioxide, and gold disrupt the gut microbiota, damage the intestinal barrier, and activate the immune system, elevating pro-inflammatory cytokines such as IL-15, IFN-γ, and IL-8. Exposure to heavy metals is associated with over-representation of Bacteroides and Firmicutes, impaired tight junctions, and disrupted autophagy in enterocytes, all promoting intestinal injury. Individuals on gluten-free diets, especially those rich in rice-based products, may accumulate more heavy metals, exacerbating mucosal damage. Likewise, pollutants like nitrogen dioxide, particulate matter, and ozone weaken gut integrity, alter immune responses, and are linked to increased coeliac disease prevalence, particularly in children in polluted areas.
Summary:
Heavy metals and air pollution may alter the intestinal microenvironment, epithelial barrier function, and immune response, representing potential risk factors for the development of coeliac disease. Their role in the disease’s pathogenesis warrants further multicentre, multidisciplinary research.
REFERENCJE (42)
1.
Patel N, Robert ME. Frontiers in celiac disease: Where autoimmunity and environment meet. Am J Surg Pathol. 2022;46(1):e43–e54. https://doi.org/10.1097/PAS.00....
2.
Crawley C, Sander SD, Nohr EA, et al. Early environmental risk factors and coeliac disease in adolescents: a population-based cohort study in Denmark. BMJ Open. 2023;13(11):e061006. https://doi.org/10.1136/bmjope....
3.
Galipeau HJ, Hinterleitner R, Leonard MM, Caminero A. Non-host factors influencing onset and severity of celiac disease. Gastroenterol. 2024;167(1):34–50. https://doi.org/10.1053/j.gast....
4.
Skoracka K, Hryhorowicz S, Rychter AM, et al. Why are western diet and western lifestyle pro-inflammatory risk factors of celiac disease? Front Nutr. 2023;9:1054089. https://doi.org/10.3389/fnut.2....
5.
Farrier CE, Wanat M, Harnden A, et al. Predictive factors for the diagnosis of coeliac disease in children and young people in primary care: A systematic review and meta-analysis. PLoS One. 2024;19(12):e0306844. https://doi.org/10.1371/journa....
6.
Catassi C, Verdu EF, Bai JC, Lionetti E. Coeliac disease. Lancet. 2022;399(10344):2413–2426. https://doi.org/10.1016/S0140-....
7.
Wessels M, Auricchio R, Dolinsek J, et al. Review on pediatric coeliac disease from a clinical perspective. Eur J Pediatr. 2022;181:1785–1795. https://doi.org/10.1007/s00431....
8.
Aboulaghras S, Piancatelli D, Oumhani K, Balahbib A, Bouyahya A, et al. Pathophysiology and immunogenetics of celiac disease. Clin Chim Acta. 2022;528:74–83. https://doi.org/10.1016/j.cca.....
9.
Ge HJ, Chen XL. Advances in understanding and managing celiac disease: Pathophysiology and treatment strategies. World J Gastroenterol. 2024;30(35):3932–3941. https://doi.org/10.3748/wjg.v3....
10.
Hudec M, Riegerová K, Pala J, et al. Celiac disease defined by over‑sensitivity to gliadin activation and superior antigen presentation of dendritic cells. Int J Mol Sci. 2021;22(18):9982. https://doi.org/10.3390/ijms22....
11.
Shiha MG, Chetcuti Zammit S, et al. Updates in the diagnosis and management of coeliac disease. Best Pract Res Clin Gastroenterol. 2023;64–65:101843. https://doi.org/10.1016/j.bpg.....
12.
Wu X, Qian L, Liu K, et al. Gastrointestinal microbiome and gluten in celiac disease. Ann Med. 2021;53(1):1797–1805. https://doi.org/10.1080/078538....
13.
Kinashi Y, Hase K. Partners in leaky gut syndrome: intestinal dysbiosis and autoimmunity. Front Immunol. 2021;12:673708. https://doi.org/10.3389/fimmu.....
14.
Husby S, Koletzko S, Korponay-Szabó I, et al. European Society Paediatric Gastroenterology, Hepatology and Nutrition guidelines for diagnosing coeliac disease. J Pediatr Gastroenterol Nutr. 2020;70(1):141–156. https://doi.org/10.1097/MPG.00....
15.
Husby S, Murray JA, Katzka DA. AGA clinical practice update on diagnosis and monitoring of celiac disease—changing utility of serology and histologic measures: expert review. Gastroenterol. 2019;156(4):885–889. https://doi.org/10.1053/j.gast....
16.
Al-Toma A, Volta U, Auricchio R, et al. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United Eur Gastroenterol J. 2019;7(5):583–613. https://doi.org/10.1177/205064....
17.
Previtali G, Licini L, D'Antiga L, et al. Celiac disease diagnosis without biopsy: is a 10×ULN antitransglutaminase result suitable for a chemiluminescence method? J Pediatr Gastroenterol Nutr. 2018;66(3):417–422. https://doi.org/10.1097/MPG.00....
18.
Werkstetter KJ, Korponay-Szabó IR, Popp A, et al. Accuracy in diagnosis of celiac disease without biopsies in clinical practice. Gastroenterol. 2017;153(4):924–935. https://doi.org/10.1053/j.gast....
19.
Leonard MM, Lebwohl B, Rubio-Tapia A, Biagi F. AGA clinical practice update on the evaluation and management of seronegative enteropathies: expert review. Gastroenterol. 2021;160(4):1288–1297. https://doi.org/10.1053/j.gast....
20.
Dunne MR, Byrne G, Chirdo FG, Feighery C. Coeliac disease pathogenesis: The uncertainties of a well-known immune-mediated disorder. Front Immunol. 2020;11:1374. https://doi.org/10.3389/fimmu.....
21.
Therrien A, Kelly CP, Silvester JA. Celiac disease: Extraintestinal manifestations and associated conditions. J Clin Gastroenterol. 2020;54(1):8–21. https://doi.org/10.1097/MCG.00....
22.
Stefanelli G, Viscido A, Longo S, et al. Persistent iron deficiency anemia in patients with celiac disease despite a gluten‑free diet. Nutrients. 2020;12(8):2176. https://doi.org/10.3390/nu1208....
23.
Wieser H, Segura V, Ruiz-Carnicer Á, et al. Food safety and cross-contamination of gluten-free products: A narrative review. Nutrients. 2021;13(7):2244. https://doi.org/10.3390/nu1307....
24.
Lionetti E, Gatti S, Galeazzi T, et al. Safety of oats in children with celiac disease: A double-blind, randomized, placebo-controlled trial. J Pediatr. 2018;194:116–122.e2. https://doi.org/10.1016/j.jped....
25.
Di Nardo G, Villa MP, Conti L, et al. Nutritional deficiencies in children with celiac disease resulting from a gluten-free diet: A systematic review. Nutrients. 2019;11(1):158. https://doi.org/10.3390/nu1101....
26.
Lionetti E, Antonucci N, Marinelli M, et al. Nutritional status, dietary intake, and adherence to the Mediterranean diet of children with celiac disease on a gluten-free diet: A case-control prospective study. Nutrients. 2020;12(5):1431. https://doi.org/10.3390/nu1205....
27.
Stein AC, Liao C, Paski S, et al. Obesity and cardiovascular risk in adults with celiac disease. J Clin Gastroenterol. 2016;50(7):545–550. https://doi.org/10.1097/MCG.00....
28.
Nazareth S, Lebwohl B, Tennyson CA, et al. Dietary supplement use in patients with celiac disease in the United States. J Clin Gastroenterol. 2015;49(9):745–751. https://doi.org/10.1097/MCG.00....
29.
Al-Toma A, Volta U, Auricchio R, et al. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United Eur Gastroenterol J. 2019;7(5):583–614. https://doi.org/10.1177/205064....
30.
Hujoel IA, Murray JA. Refractory celiac disease. Curr Gastroenterol Rep. 2020;22(10):48. https://doi.org/10.1007/s11894....
31.
Ghosh S, Nukavarapu SP, Jala VR. Effects of heavy metals on gut barrier integrity and gut microbiota. Microbiota Host. 2024;2(1):1. https://doi.org/10.1530/MAH-23....
32.
Elliott KA, Rinna CR, Le Gall G. Nanoparticles in the food industry and their impact on the human gut microbiome. Nanotoxicology Gut Health. 2021;1(1):1.
33.
Du N, Chang D, Boisvert J, et al. Effect of adopting a gluten‑free diet on exposure to arsenic and other heavy metals in children with celiac disease: a prospective cohort study. Am J Gastroenterol. 2024;120(4):883–889. https://doi.org/10.14309/ajg.0....
34.
Mancuso C, Re F, Rivolta I, et al. Dietary nanoparticles interact with gluten peptides and alter the intestinal homeostasis increasing the risk of celiac disease. Int J Mol Sci. 2021;22(11):6102. https://doi.org/10.3390/ijms22....
35.
Lu X, Feng R, Liu L, et al. Identifying celiac disease‑related chemicals by transcriptome‑wide association study and chemical–gene interaction analyses. Front Genet. 2022;13:990483. https://doi.org/10.3389/fgene.....
36.
Bascuñán KA, Orosteguí C, Rodríguez JM, et al. Heavy metal and rice in gluten‑free diets: are they a risk? Nutrients. 2023;15(13):2975. https://doi.org/10.3390/nu1503....
37.
King JA, Jeong J, Underwood FE, et al. Incidence of celiac disease is increasing over time: a systematic review and meta-analysis. Am J Gastroenterol. 2020;115(4):507–525. https://doi.org/10.14309/ajg.0....
38.
Gaylord A, Trasande L, Kannan K, et al. Persistent organic pollutant exposure and celiac disease: A pilot study. Environ Res. 2020;186:109439. https://doi.org/10.1016/j.envr....
39.
Leonard MM, Karathia H, Pujolassos M, et al. Multi-omics analysis reveals the influence of genetic and environmental risk factors on developing gut microbiota in infants at risk of celiac disease. Microbiome. 2020;8:130. https://doi.org/10.1186/s40168....
40.
Salim SY, Kaplan GG, Madsen KL. Air pollution effects on the gut microbiota: A link between exposure and inflammatory disease. Gut Microbes. 2013;5(2):215–219. https://doi.org/10.4161/gmic.2....
41.
Pujolassos M, Leonard MM, Colucci A, Fasano A. Environment and the microbiome in early life: the key to celiac disease development? Gut Microbes. 2021;13(1):1–16. https://doi.org/10.1080/194909....
42.
Chen H, Burnett RT, Kwong JC, et al. Risk of incident autoimmune diseases associated with air pollution exposure: A population-based cohort study. Environ Int. 2022;157:106842. https://doi.org/10.1016/j.envi....
Udostępnij
ARTYKUŁ POWIĄZANY
Przetwarzamy dane osobowe zbierane podczas odwiedzania serwisu. Realizacja funkcji pozyskiwania informacji o użytkownikach i ich zachowaniu odbywa się poprzez dobrowolnie wprowadzone w formularzach informacje oraz zapisywanie w urządzeniach końcowych plików cookies (tzw. ciasteczka). Dane, w tym pliki cookies, wykorzystywane są w celu realizacji usług, zapewnienia wygodnego korzystania ze strony oraz w celu monitorowania ruchu zgodnie z Polityką prywatności. Dane są także zbierane i przetwarzane przez narzędzie Google Analytics (więcej).
Możesz zmienić ustawienia cookies w swojej przeglądarce. Ograniczenie stosowania plików cookies w konfiguracji przeglądarki może wpłynąć na niektóre funkcjonalności dostępne na stronie.
Możesz zmienić ustawienia cookies w swojej przeglądarce. Ograniczenie stosowania plików cookies w konfiguracji przeglądarki może wpłynąć na niektóre funkcjonalności dostępne na stronie.